Introduction
This study aimed to investigate the underlying reasons behind sustained significant continuous deep fluctuations in the absolute lymphocyte count (ALC) in an untreated patient with Chronic Lymphocytic Leukemia (CLL), who has maintained a favorable prognosis since the time of diagnosis in 2016. The patient has voluntarily chosen to adopt a mainly vegetarian and fruitarian diet, including annual periods of prolonged total fasting (lasting from 4 to 39 days).
Methods
For this purpose, we decided to conduct a comprehensive analysis of the whole transcriptome profiling of peripheral blood (PB) CD19+ cells from patient (#1) at different time-points, in comparison to the corresponding cells from 5 other untreated CLL patients who followed a varied diet. As a result, the CLL patients were classified as follows: the 1st group consisted of patient #1 at 20 different time-points (16 time-points during nutrition and 4 time-points during fasting), whereas the 2nd group included only one time point for each of the patients (#2, #3, #4, #5, and #6) due to their varied dietary practices.
We have performed microarray experiments on the Peripheral Bone Marrow Cells (PBMCs) CD19+ cells utilizing the Affymetrix Human Clariom™ D Pico Assay, a powerful tool for generating high-fidelity biomarker signatures.
The data was preprocessed and normalized using the Robust Multiarray Average (RMA) method. Significance Analysis of Microarrays (SAM version 3.0) was utilized to assess significant differentially expressed genes between the 1st group and 2nd group of CLL patients. The Storey and Tibshirani procedure was applied to control the False Discovery Rate (FDR).
Results
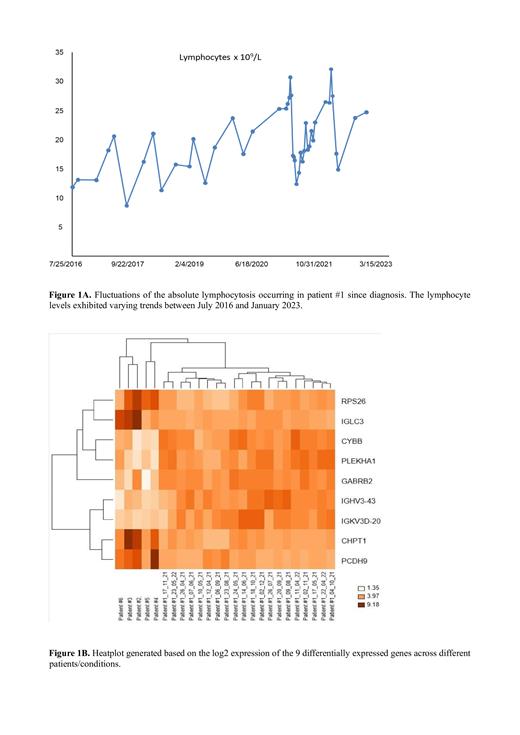
The lymphocytosis trend of patient #1 displayed recurring significant deep fluctuations since the time of diagnosis. Interestingly, we observed that approximately 4-6 weeks after the conclusion of fasting periods, the absolute lymphocyte count was reduced by about half (Figure 1A). On the other hand, in the 2nd group, all patients did not show fluctuations in the absolute lymphocyte count, but instead demonstrated a progressive increase in lymphocytes over time.
Differential expression analysis, after correction for multiple testing, identified 18 differentially expressed genes (DEGs) in the comparison between CD19+ cells of patient #1 and patients #2, #3, #4, #5, and #6. Among them, our focus was on 9 genes with functional annotation: 4 genes were downregulated ( IGLC3, RPS26, CHPT1, and PCDH9), and 5 genes were upregulated ( IGHV3-43, IGKV3D-20, PLEKHA1, CYBB, and GABRB2) in the 1st group (patient #1) vs. the 2nd group of patients (#2, #3, #4, #5, and #6), respectively. In particular, IGLC3, RPS26, PLEKHA1, CYBB, and GABRB2 showed high Fold Change (FC) > 3. Additionally, we generated a heat map on log2 expression of the 9 differentially expressed genes across different patients/conditions (Figure 1B).
Furthermore, hierarchical clustering analysis revealed that patient #1 during nutrition and fasting (1st group) clustered distinctly from all CLL patients (2nd group), indicating a gene expression signature that differentiated patient #1 from patient #2, #3, #4, #5, and #6.
Conclusions
In conclusion, our transcriptomic study revealed a small set of 9 genes that distinguished an untreated CLL patient who underwent a prolonged periods of total fasting and maintained a slow-growing tendency of lymphocytosis, in comparison to 5 untreated CLL patients with a varied diet. Future investigations of patient #1 could demonstrate the potential role of prolonged periodic fasting and the involvement of the 9 genes in sustaining the trend of lymphocytosis and the benign course of the disease.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal